Magnetic resonance imaging
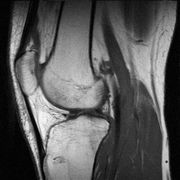
Magnetic resonance imaging (MRI), or nuclear magnetic resonance imaging (NMRI), is primarily a noninvasive medical imaging technique used in radiology to visualize detailed internal structure and limited function of the body. MRI provides much greater contrast between the different soft tissues of the body than computed tomography (CT) does, making it especially useful in neurological (brain), musculoskeletal, cardiovascular, and oncological (cancer) imaging.
Unlike CT, MRI uses no ionizing radiation. Rather, it uses a powerful magnetic field to align the nuclear magnetization of (usually) hydrogen atoms in water in the body. Radio frequency (RF) fields are used to systematically alter the alignment of this magnetization. This causes the hydrogen nuclei to produce a rotating magnetic field detectable by the scanner. This signal can be manipulated by additional magnetic fields to build up enough information to construct an image of the body.[1]:36
Magnetic resonance imaging is a relatively new technology. The first MR image was published in 1973[2][3] and the first cross-sectional image of a living mouse was published in January 1974.[4] The first studies performed on humans were published in 1977.[5][6] By comparison, the first human X-ray image was taken in 1895.
Magnetic resonance imaging is a development of nuclear magnetic resonance. Originally, the technique was referred to as nuclear magnetic resonance imaging (NMRI). However, because the word nuclear was associated in the public mind with ionizing radiation exposure, it is generally now referred to simply as MRI. Scientists still use the term NMRI when discussing non-medical devices operating on the same principles. The term magnetic resonance tomography (MRT) is also sometimes used.
How MRI works
The body is largely composed of water molecules. Each water molecule has two hydrogen nuclei or protons. When a person goes inside the powerful magnetic field of the scanner, the magnetic moments of some of these protons changes, and aligns with the direction of the field.
In an MRI machine a radio frequency transmitter is briefly turned on, producing an electromagnetic field. The photons of this field have just the right energy, known as the resonance frequency, to flip the spin of the aligned protons in the body. As the intensity and duration of application of the field increase, more aligned spins are affected. After the field is turned off, the protons decay to the original spin-down state and the difference in energy between the two states is released as a photon. It is these photons that produce the electromagnetic signal that the scanner detects. The frequency the protons resonate at depends on the strength of the magnetic field. As a result of conservation of energy, this also dictates the frequency of the released photons. The photons released when the field is removed have an energy — and therefore a frequency — due to the amount of energy the protons absorbed while the field was active.
It is this relationship between field-strength and frequency that allows the use of nuclear magnetic resonance for imaging. Additional magnetic fields are applied during the scan to make the magnetic field strength depend on the position within the patient, providing a straightforward method to control where the protons are excited by the radio photons. These fields are created by passing electric currents through solenoids, known as gradient coils. Since these coils are within the bore of the scanner, there are large forces between them and the main field coils, producing most of the noise that is heard during operation. Without efforts to dampen this noise, it can approach 130 decibels (dB) with strong fields [7] (see also #Acoustic_noise).
An image can be constructed because the protons in different tissues return to their equilibrium state at different rates, which is a difference that can be detected. By changing the parameters on the scanner, this effect is used to create contrast between different types of body tissue or between other properties, as in fMRI and diffusion MRI.
Contrast agents may be injected intravenously to enhance the appearance of blood vessels, tumors or inflammation. Contrast agents may also be directly injected into a joint in the case of arthrograms, MRI images of joints. Unlike CT, MRI uses no ionizing radiation and is generally a very safe procedure. Nonetheless the strong magnetic fields and radio pulses can affect metal implants, including cochlear implants and cardiac pacemakers. In the case of cardiac pacemakers, the results can sometimes be lethal,[8] so patients with such implants are generally not eligible for MRI.
MRI is used to image every part of the body, and is particularly useful for tissues with many hydrogen nuclei and little density contrast, such as the brain, muscle, connective tissue and most tumors.
Applications
In clinical practice, MRI is used to distinguish pathologic tissue (such as a brain tumor) from normal tissue. One advantage of an MRI scan is that it is believed to be harmless to the patient. It uses strong magnetic fields and non-ionizing radiation in the radio frequency range, unlike CT scans and traditional X-rays, which both use ionizing radiation.
While CT provides good spatial resolution (the ability to distinguish two separate structures an arbitrarily small distance from each other), MRI provides comparable resolution with far better contrast resolution (the ability to distinguish the differences between two arbitrarily similar but not identical tissues). The basis of this ability is the complex library of pulse sequences that the modern medical MRI scanner includes, each of which is optimized to provide image contrast based on the chemical sensitivity of MRI.
For example, with particular values of the echo time (TE) and the repetition time (TR), which are basic parameters of image acquisition, a sequence takes on the property of T2-weighting. On a T2-weighted scan, water- and fluid-containing tissues are bright (most modern T2 sequences are actually fast T2 sequences) and fat-containing tissues are dark. The reverse is true for T1-weighted images. Damaged tissue tends to develop edema, which makes a T2-weighted sequence sensitive for pathology, and generally able to distinguish pathologic tissue from normal tissue. With the addition of an additional radio frequency pulse and additional manipulation of the magnetic gradients, a T2-weighted sequence can be converted to a FLAIR sequence, in which free water is now dark, but edematous tissues remain bright. This sequence in particular is currently the most sensitive way to evaluate the brain for demyelinating diseases, such as multiple sclerosis.
The typical MRI examination consists of 5–20 sequences, each of which are chosen to provide a particular type of information about the subject tissues. This information is then synthesized by the interpreting physician.
Basic MRI scans
T1-weighted MRI
T1-weighted scans use a gradient echo (GRE) sequence, with short TE and short TR. This is one of the basic types of MR contrast and is a commonly run clinical scan. The T1 weighting can be increased (improving contrast) with the use of an inversion pulse as in an MP-RAGE sequence. Due to the short repetition time (TR) this scan can be run very fast allowing the collection of high resolution 3D datasets. A T1 reducing gadolinium contrast agent is also commonly used, with a T1 scan being collected before and after administration of contrast agent to compare the difference. In the brain T1-weighted scans provide good gray matter/white matter contrast, in other words put SIMPLY, T1 Weighted Images highlights fat deposition.
T2-weighted MRI
T2-weighted scans use a spin echo (SE) sequence, with long TE and long TR. They have long been the clinical workhorse as the spin echo sequence is less susceptible to inhomogeneities in the magnetic field. They are particularly well suited to edema as they are sensitive to water content (edema is characterized by increased water content). In other words, put more simply, T2 weighted images light up liquid on the images being visualized.
T*2-weighted MRI
T*2 (pronounced "T 2 star") weighted scans use a gradient echo (GRE) sequence, with long TE and long TR. The gradient echo sequence used does not have the extra refocusing pulse used in spin echo so it is subject to additional losses above the normal T2 decay (referred to as T2′), these taken together are called T*2. This also makes it more prone to susceptibility losses at air/tissue boundaries, but can increase contrast for certain types of tissue, such as venous blood.
Spin density weighted MRI
Spin density, also called proton density, weighted scans try to have no contrast from either T2 or T1 decay, the only signal change coming from differences in the amount of available spins (hydrogen nuclei in water). It uses a spin echo or sometimes a gradient echo sequence, with short TE and long TR.
Specialized MRI scans
Diffusion MRI
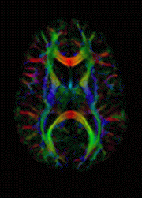
Diffusion MRI measures the diffusion of water molecules in biological tissues.[9] In an isotropic medium (inside a glass of water for example) water molecules naturally move randomly according to turbulence and Brownian motion. In biological tissues however, where the Reynold's number is low enough for flows to be laminar, the diffusion may be anisotropic. For example a molecule inside the axon of a neuron has a low probability of crossing the myelin membrane. Therefore the molecule moves principally along the axis of the neural fiber. If we know that molecules in a particular voxel diffuse principally in one direction we can make the assumption that the majority of the fibers in this area are going parallel to that direction.
The recent development of diffusion tensor imaging (DTI)[3] enables diffusion to be measured in multiple directions and the fractional anisotropy in each direction to be calculated for each voxel. This enables researchers to make brain maps of fiber directions to examine the connectivity of different regions in the brain (using tractography) or to examine areas of neural degeneration and demyelination in diseases like Multiple Sclerosis.
Another application of diffusion MRI is diffusion-weighted imaging (DWI). Following an ischemic stroke, DWI is highly sensitive to the changes occurring in the lesion.[10] It is speculated that increases in restriction (barriers) to water diffusion, as a result of cytotoxic edema (cellular swelling), is responsible for the increase in signal on a DWI scan. The DWI enhancement appears within 5–10 minutes of the onset of stroke symptoms (as compared with computed tomography, which often does not detect changes of acute infarct for up to 4–6 hours) and remains for up to two weeks. Coupled with imaging of cerebral perfusion, researchers can highlight regions of "perfusion/diffusion mismatch" that may indicate regions capable of salvage by reperfusion therapy.
Like many other specialized applications, this technique is usually coupled with a fast image acquisition sequence, such as echo planar imaging sequence.
Magnetization Transfer MRI
Magnetization transfer (MT) refers to the transfer of longitudinal magnetization from free water protons to hydration water protons in NMR and MRI.
In magnetic resonance imaging of molecular solutions, such as protein solutions, two types of water molecules, free (bulk) and hydration (bound), are found. Free water protons have faster average rotational frequency and hence less fixed water molecules that may cause local field inhomogeneity. Because of this uniformity, most free water protons have resonance frequency lying narrowly around the normal proton resonance frequency of 63 MHz (at 1.5 teslas). This also results in slower transverse magnetization dephasing and hence longer T2. Conversely, hydration water molecules are slowed down by interaction with solute molecules and hence create field inhomogeneities that lead to wider resonance frequency spectrum.
Fluid attenuated inversion recovery (FLAIR)
Fluid Attenuated Inversion Recovery (FLAIR)[11] is an inversion-recovery pulse sequence used to null signal from fluids. For example, it can be used in brain imaging to suppress cerebrospinal fluid (CSF) so as to bring out the periventricular hyperintense lesions, such as multiple sclerosis (MS) plaques. By carefully choosing the inversion time TI (the time between the inversion and excitation pulses), the signal from any particular tissue can be suppressed.
Magnetic resonance angiography

Magnetic resonance angiography (MRA) generates pictures of the arteries to evaluate them for stenosis (abnormal narrowing) or aneurysms (vessel wall dilatations, at risk of rupture). MRA is often used to evaluate the arteries of the neck and brain, the thoracic and abdominal aorta, the renal arteries, and the legs (called a "run-off"). A variety of techniques can be used to generate the pictures, such as administration of a paramagnetic contrast agent (gadolinium) or using a technique known as "flow-related enhancement" (e.g. 2D and 3D time-of-flight sequences), where most of the signal on an image is due to blood that recently moved into that plane, see also FLASH MRI. Techniques involving phase accumulation (known as phase contrast angiography) can also be used to generate flow velocity maps easily and accurately. Magnetic resonance venography (MRV) is a similar procedure that is used to image veins. In this method, the tissue is now excited inferiorly, while signal is gathered in the plane immediately superior to the excitation plane—thus imaging the venous blood that recently moved from the excited plane.[12]
Magnetic resonance gated intracranial CSF dynamics (MR-GILD)
Magnetic resonance gated intracranial cerebrospinal fluid (CSF) or liquor dynamics (MR-GILD) technique is an MR sequence based on bipolar gradient pulse used to demonstrate CSF pulsatile flow in ventricles, cisterns, aqueduct of Sylvius and entire intracranial CSF pathway. It is a method for analyzing CSF circulatory system dynamics in patients with CSF obstructive lesions such as normal pressure hydrocephalus. It also allows visualization of both arterial and venous pulsatile blood flow in vessels without use of contrast agents.[13][14]
| Diastolic time data acquisition (DTDA). | Systolic time data acquisition (STDA). |
|---|---|
 |
 |
Magnetic resonance spectroscopy
Magnetic resonance spectroscopy (MRS) is used to measure the levels of different metabolites in body tissues. The MR signal produces a spectrum of resonances that correspond to different molecular arrangements of the isotope being "excited". This signature is used to diagnose certain metabolic disorders, especially those affecting the brain,[15] and to provide information on tumor metabolism.[16]
Magnetic resonance spectroscopic imaging (MRSI) combines both spectroscopic and imaging methods to produce spatially localized spectra from within the sample or patient. The spatial resolution is much lower (limited by the available SNR), but the spectra in each voxel contains information about many metabolites. Because the available signal is used to encode spatial and spectral information, MRSI requires high SNR achievable only at higher field strengths (3 T and above).
Functional MRI

Functional MRI (fMRI) measures signal changes in the brain that are due to changing neural activity. The brain is scanned at low resolution but at a rapid rate (typically once every 2–3 seconds). Increases in neural activity cause changes in the MR signal via T*2 changes;[17] this mechanism is referred to as the BOLD (blood-oxygen-level dependent) effect. Increased neural activity causes an increased demand for oxygen, and the vascular system actually overcompensates for this, increasing the amount of oxygenated hemoglobin relative to deoxygenated hemoglobin. Because deoxygenated hemoglobin attenuates the MR signal, the vascular response leads to a signal increase that is related to the neural activity. The precise nature of the relationship between neural activity and the BOLD signal is a subject of current research. The BOLD effect also allows for the generation of high resolution 3D maps of the venous vasculature within neural tissue.
While BOLD signal is the most common method employed for neuroscience studies in human subjects, the flexible nature of MR imaging provides means to sensitize the signal to other aspects of the blood supply. Alternative techniques employ arterial spin labeling (ASL) or weight the MRI signal by cerebral blood flow (CBF) and cerebral blood volume (CBV). The CBV method requires injection of a class of MRI contrast agents that are now in human clinical trials. Because this method has been shown to be far more sensitive than the BOLD technique in preclinical studies, it may potentially expand the role of fMRI in clinical applications. The CBF method provides more quantitative information than the BOLD signal, albeit at a significant loss of detection sensitivity.
Interventional MRI
The lack of harmful effects on the patient and the operator make MRI well-suited for "interventional radiology", where the images produced by a MRI scanner are used to guide minimally invasive procedures. Of course, such procedures must be done without any ferromagnetic instruments.
A specialized growing subset of interventional MRI is that of intraoperative MRI in which the MRI is used in the surgical process. Some specialized MRI systems have been developed that allow imaging concurrent with the surgical procedure. More typical, however, is that the surgical procedure is temporarily interrupted so that MR images can be acquired to verify the success of the procedure or guide subsequent surgical work.
Radiation therapy simulation
Because of MRI's superior imaging of soft tissues, it is now being utilized to specifically locate tumors within the body in preparation for radiation therapy treatments. For therapy simulation, a patient is placed in specific, reproducible, body position and scanned. The MRI system then computes the precise location, shape and orientation of the tumor mass, correcting for any spatial distortion inherent in the system. The patient is then marked or tattooed with points that, when combined with the specific body position, permits precise triangulation for radiation therapy.
Current density imaging
Current density imaging (CDI) endeavors to use the phase information from images to reconstruct current densities within a subject. Current density imaging works because electrical currents generate magnetic fields, which in turn affect the phase of the magnetic dipoles during an imaging sequence. To date no successful CDI has been performed using biological currents, but several studies have been published that involve currents applied through a pair of electrodes.
Magnetic resonance guided focused ultrasound
In MRgFUS therapy, ultrasound beams are focused on a tissue—guided and controlled using MR thermal imaging—and due to the significant energy deposition at the focus, temperature within the tissue rises to more than 65 °C (150 °F), completely destroying it. This technology can achieve precise "ablation" of diseased tissue. MR imaging provides a three-dimensional view of the target tissue, allowing for precise focusing of ultrasound energy. The MR imaging provides quantitative, real-time, thermal images of the treated area. This allows the physician to ensure that the temperature generated during each cycle of ultrasound energy is sufficient to cause thermal ablation within the desired tissue and if not, to adapt the parameters to ensure effective treatment.
Multinuclear imaging
Hydrogen is the most frequently imaged nucleus in MRI because it is present in biological tissues in great abundance. However, any nucleus with a net nuclear spin could potentially be imaged with MRI. Such nuclei include helium-3, carbon-13, fluorine-19, oxygen-17, sodium-23, phosphorus-31 and xenon-129. 23Na, 31P and 17O are naturally abundant in the body, so can be imaged directly. Gaseous isotopes such as 3He or 129Xe must be hyperpolarized and then inhaled as their nuclear density is too low to yield a useful signal under normal conditions. 17O, 13C and 19F can be administered in sufficient quantities in liquid form (e.g. 17O-water, 13C-glucose solutions or perfluorocarbons) that hyperpolarization is not a necessity.
Multinuclear imaging is primarily a research technique at present. However, potential applications include functional imaging and imaging of organs poorly seen on 1H MRI (e.g. lungs and bones) or as alternative contrast agents. Inhaled hyperpolarized 3He can be used to image the distribution of air spaces within the lungs. Injectable solutions containing 13C or stabilized bubbles of hyperpolarized 129Xe have been studied as contrast agents for angiography and perfusion imaging. 31P can potentially provide information on bone density and structure, as well as functional imaging of the brain.
Susceptibility weighted imaging (SWI)
Susceptibility weighted imaging (SWI), is a new type of contrast in MRI different from spin density, T1, or T2 imaging. This method exploits the susceptibility differences between tissues and uses a fully velocity compensated, three dimensional, RF spoiled, high-resolution, 3D gradient echo scan. This special data acquisition and image processing produces an enhanced contrast magnitude image very sensitive to venous blood, hemorrhage and iron storage. It is used to enhance the detection and diagnosis of tumors, vascular and neurovascular diseases (stroke and hemorrhage, multiple sclerosis, Alzheimer's), and also detects traumatic brain injuries that may not be diagnosed using other methods"Radiology". http://radiology.rsna.org/content/204/1/272.long. Retrieved 2 August 2010. (subscription required)</ref></ref>
Other specialized MRI techniques
MRI is a new and active field of research and new methods and variants are often published when they are able to get better results in specific fields. Examples of these recent improvements are T*2-weighted turbo spin-echo (T2 TSE MRI), double inversion recovery MRI (DIR-MRI) or phase-sensitive inversion recovery MRI (PSIR-MRI), all of them able to improve imaging of the brain lesions.[18][19] Another example is MP-RAGE (magnetization-prepared rapid acquisition with gradient echo),[20] which improves images of multiple sclerosis cortical lesions.[21]
Portable instruments
Portable magnetic resonance instruments are available for use in education and field research. Using the principles of Earth's field NMR, they have no powerful polarizing magnet, so that such instruments can be small and inexpensive. Some can be used for both EFNMR spectroscopy and MRI imaging.[22] The low strength of the Earth's field results in poor signal to noise ratios, requiring long scan times to capture spectroscopic data or build up MRI images.
Research with atomic magnetometers have discussed the possibility for cheap and portable MRI instruments without the large magnet.[23][24]
MRI versus CT
A computed tomography (CT) scanner uses X-rays, a type of ionizing radiation, to acquire its images, making it a good tool for examining tissue composed of elements of a higher atomic number than the tissue surrounding them, such as bone and calcifications (calcium based) within the body (carbon based flesh), or of structures (vessels, bowel). MRI, on the other hand, uses non-ionizing radio frequency (RF) signals to acquire its images and is best suited for non-calcified tissue, though MR images can also be acquired from bones and teeth[25] as well as fossils.[26]
CT may be enhanced by use of contrast agents containing elements of a higher atomic number than the surrounding flesh such as iodine or barium. Contrast agents for MRI have paramagnetic properties, e.g., gadolinium and manganese.
Both CT and MRI scanners are able to generate multiple two-dimensional cross-sections (slices) of tissue and three-dimensional reconstructions. Unlike CT, which uses only X-ray attenuation to generate image contrast, MRI has a long list of properties that may be used to generate image contrast. By variation of scanning parameters, tissue contrast can be altered and enhanced in various ways to detect different features. (See Applications above.)
MRI can generate cross-sectional images in any plane (including oblique planes). In the past, CT was limited to acquiring images in the axial (or near axial) plane. The scans used to be called Computed Axial Tomography scans (CAT scans). However, the development of multi-detector CT scanners with near-isotropic resolution, allows the CT scanner to produce data that can be retrospectively reconstructed in any plane with minimal loss of image quality.
For purposes of tumor detection and identification in the brain, MRI is generally superior.[27][28][29] However, in the case of solid tumors of the abdomen and chest, CT is often preferred due to less motion artifact. Furthermore, CT usually is more widely available, faster, less expensive, and may be less likely to require the person to be sedated or anesthetized.
MRI is also best suited for cases when a patient is to undergo the exam several times successively in the short term, because, unlike CT, it does not expose the patient to the hazards of ionizing radiation.
Economics of MRI
MRI equipment is expensive. 1.5 tesla scanners often cost between $1 million and $1.5 million USD. 3.0 tesla scanners often cost between $2 million and $2.3 million USD. Construction of MRI suites can cost up to $500,000 USD, or more, depending on project scope.
MRI scanners have been significant sources of revenue for healthcare providers in the US. This is because of favorable reimbursement rates from insurers and federal government programs. Insurance reimbursement is provided in two components, an equipment charge for the actual performance of the MRI scan and professional charge for the radiologist's review of the images and/or data. In the US Northeast, an equipment charge might be $3,500 and a professional charge might be $350 [30] although the actual fees received by the equipment owner and interpreting physician are often significantly less and depend on the rates negotiated with insurance companies or determined by governmental action as in the Medicare Fee Schedule. For example, an orthopedic surgery group in Illinois billed a charge of $1,116 for a knee MRI in 2007 but the Medicare reimbursement in 2007 was only $470.91.[31] Many insurance companies require preapproval of an MRI procedure as a condition for coverage.
In the US, the Deficit Reduction Act of 2007 significantly reduced reimbursement rates paid by federal insurance programs for the equipment component of many scans, shifting the economic landscape. Many private insurers have followed suit.
Installation of the MRI unit

An MRI unit is a rather large item, typically requiring heavy equipment (such as cranes) to move the unit to its final location. Once the MRI unit is in place, the room that houses it is usually "built up" around the unit itself. See this page for an example of the complexity involved in installing an MRI unit in a clinical setting.[32]
Safety
Death and injuries have occurred from projectiles created by the magnetic field, although few compared to the millions of examinations administered.[33][34] MRI makes use of powerful magnetic fields that, though not known to cause direct biological damage, can interfere with metallic and electromechanical devices. Additional (small) risks are presented by the radio frequency systems, components or elements of the MRI system's operation, elements of the scanning procedure and medications that may be administered to facilitate MRI imaging.
Of great concern is the dramatic increase in the number of reported MRI accidents to the U.S. Food and Drug Administration (FDA). Since 2004, the last year in which a decline in the number of MRI accidents was reported, the full spectrum of MRI accidents has increased significantly in the following years. The 2008 FDA accident report data culminates in a 277% increase over the 2004 rate.
There are many steps that the MRI patient and referring physician can take to help reduce the remaining risks, including providing a full, accurate and thorough medical history to the MRI provider.
Several of the specific MRI safety considerations are identified below:
Implants and foreign bodies
Pacemakers are generally considered an absolute contraindication towards MRI scanning, though highly specialized protocols have been developed to permit scanning of select pacing devices. Several cases of arrhythmia or death have been reported in patients with pacemakers who have undergone MRI scanning without appropriate precautions. Other electronic implants have varying contraindications, depending upon scanner technology, and implant properties, scanning protocols and anatomy being imaged.
Many other forms of medical or biostimulation implants may be contraindicated for MRI scans. These may include vagus nerve stimulators, implantable cardioverter-defibrillators, loop recorders, insulin pumps, cochlear implants, deep brain stimulators, and many others. Medical device patients should always present complete information (manufacturer, model, serial number and date of implantation) about all implants to both the referring physician and to the radiologist or technologist before entering the room for the MRI scan.
While these implants pose a current problem, scientists and manufacturers are working on improved designs that reduce risks to medical device operations. One such development in the works is a nano-coating for implants intended to screen them from the radio frequency waves, helping to make MRI exams available to patients currently prohibited from receiving them. The current article for this is from New Scientist.
Ferromagnetic foreign bodies (e.g. shell fragments), or metallic implants (e.g. surgical prostheses, aneurysm clips) are also potential risks, and safety aspects need to be considered on an individual basis. Interaction of the magnetic and radio frequency fields with such objects can lead to trauma due to movement of the object in the magnetic field, thermal injury from radio-frequency induction heating of the object, or failure of an implanted device. These issues are especially problematic when dealing with the eye. Most MRI centers require an orbital x-ray to be performed on anyone suspected of having metal fragments in their eyes, something not uncommon in metalworking.
Because of its non-ferromagnetic nature and poor electrical conductivity, titanium and its alloys are useful for long term implants and surgical instruments intended for use in image-guided surgery. In particular, not only is titanium safe from movement from the magnetic field, but artifacts around the implant are less frequent and less severe than with more ferromagnetic materials e.g. stainless steel. Artifacts from metal frequently appear as regions of empty space around the implant—frequently called 'black-hole artifact'. E.g. a 3 mm titanium alloy coronary stent may appear as a 5 mm diameter region of empty space on MRI, whereas around a stainless steel stent, the artifact may extend for 10–20 mm or more.
In 2006, a new classification system for implants and ancillary clinical devices has been developed by ASTM International and is now the standard supported by the US Food and Drug Administration:
-
MR-Safe — The device or implant is completely non-magnetic, non-electrically conductive, and non-RF reactive, eliminating all of the primary potential threats during an MRI procedure.
 MR Safe sign
MR Safe sign -
MR-Conditional — A device or implant that may contain magnetic, electrically conductive or RF-reactive components that is safe for operations in proximity to the MRI, provided the conditions for safe operation are defined and observed (such as 'tested safe to 1.5 teslas' or 'safe in magnetic fields below 500 gauss in strength').
 MR Conditional sign
MR Conditional sign -
MR-Unsafe — Nearly self-explanatory, this category is reserved for objects that are significantly ferromagnetic and pose a clear and direct threat to persons and equipment within the magnet room.
 MR Unsafe sign
MR Unsafe sign
Though the current classification system was originally developed for regulatory-approved medical devices, it is being applied to all manner of items, appliances and equipment intended for use in the MR environment.
In the case of pacemakers, the risk is thought to be primarily RF induction in the pacing electrodes/wires causing inappropriate pacing of the heart, rather than the magnetic field affecting the pacemaker itself. Much research and development is being undertaken, and many tools are being developed to predict RF field effects inside the body.
Projectile or missile effect
As a result of the very high strength of the magnetic field needed to produce scans (frequently up to 60,000 times the Earth's own magnetic field effects), there are several incidental safety issues addressed in MRI facilities. Missile-effect accidents, where ferromagnetic objects are attracted to the center of the magnet, have resulted in injury and death.[33][34]
To reduce the risks of projectile accidents, ferromagnetic objects and devices are typically prohibited in proximity to the MRI scanner, with non-ferromagnetic versions of many tools and devices typically retained by the scanning facility. Patients undergoing MRI examinations are required to remove all metallic objects, often by changing into a gown or scrubs.
Ferromagnetic detection devices are used by some sites as a supplement conventional screening techniques, and are now recommended by the American College of Radiology's Guidance Document for Safe MR Practices[35] and the United States Department of Veterans Affairs[36].
The magnetic field and the associated risk of missile-effect accidents remains a permanent hazard, as superconductive MRI magnets are kept permanently energized and so retain their magnetic field in the event of a power outage.
Radio frequency energy
A powerful radio transmitter is needed for excitation of proton spins. This can heat the body to the point of risk of hyperthermia in patients, particularly in obese patients or those with thermoregulation disorders. Several countries have issued restrictions on the maximum specific absorption rate that a scanner may produce.
Peripheral nerve stimulation (PNS)
The rapid switching on and off of the magnetic field gradients is capable of causing nerve stimulation. Volunteers report a twitching sensation when exposed to rapidly switched fields, particularly in their extremities. The reason the peripheral nerves are stimulated is that the changing field increases with distance from the center of the gradient coils (which more or less coincides with the center of the magnet). Note however that when imaging the head, the heart is far off-center and induction of even a tiny current into the heart must be avoided at all costs. Although PNS was not a problem for the slow, weak gradients used in the early days of MRI, the strong, rapidly switched gradients used in techniques such as EPI, fMRI, diffusion MRI, etc. are indeed capable of inducing PNS. American and European regulatory agencies insist that manufacturers stay below specified dB/dt limits (dB/dt is the change in field per unit time) or else prove that no PNS is induced for any imaging sequence. As a result of dB/dt limitation, commercial MRI systems cannot use the full rated power of their gradient amplifiers.
Acoustic noise
Switching of field gradients causes a change in the Lorentz force experienced by the gradient coils, producing minute expansions and contractions of the coil itself. As the switching is typically in the audible frequency range, the resulting vibration produces loud noises (clicking or beeping). This is most marked with high-field machines and rapid-imaging techniques in which sound intensity can reach 120 dB(A) (equivalent to a jet engine at take-off).[37] As a reference, 120 dB is the threshold of loudness causing sensation in the human ear canal — tickling, and 140 dB is the threshold of ear pain. Since decibel is a logarithmic measurement, a 10 dB increase equates to a 10-fold increase in intensity—which, in acoustics, is roughly equal to a doubling of loudness.
Appropriate use of ear protection is essential for anyone inside the MRI scanner room during the examination.[38]
Cryogens
As described above in #Scanner construction and operation, many MRI scanners rely on cryogenic liquids to enable superconducting capabilities of the electromagnetic coils within. Though the cryogenic liquids used are non-toxic, their physical properties present specific hazards.
An unintentional shut-down of a superconducting electromagnet, an event known as "quench", involves the rapid boiling of liquid helium from the device. If the rapidly expanding helium cannot be dissipated through an external vent, sometimes referred to as 'quench pipe', it may be released into the scanner room where it may cause displacement of the oxygen and present a risk of asphyxiation.[39]
Liquid helium, the most commonly used cryogen in MRI, undergoes near explosive expansion as it changes from liquid to a gaseous state. Rooms built in support of superconducting MRI equipment should be equipped with pressure relief mechanisms[40] and an exhaust fan, in addition to the required quench pipe.
Since a quench results in rapid loss of all cryogens in the magnet, recommissioning the magnet is expensive and time-consuming. Spontaneous quenches are uncommon, but may also be triggered by equipment malfunction, improper cryogen fill technique, contaminants inside the cryostat, or extreme magnetic or vibrational disturbances.
Contrast agents
The most commonly used intravenous contrast agents are based on chelates of gadolinium. In general, these agents have proved safer than the iodinated contrast agents used in X-ray radiography or CT. Anaphylactoid reactions are rare, occurring in approx. 0.03–0.1%.[41] Of particular interest is the lower incidence of nephrotoxicity, compared with iodinated agents, when given at usual doses—this has made contrast-enhanced MRI scanning an option for patients with renal impairment, who would otherwise not be able to undergo contrast-enhanced CT.[42]
Although gadolinium agents have proved useful for patients with renal impairment, in patients with severe renal failure requiring dialysis there is a risk of a rare but serious illness, nephrogenic systemic fibrosis, that may be linked to the use of certain gadolinium-containing agents. The most frequently linked is gadodiamide, but other agents have been linked too.[43] Although a causal link has not been definitively established, current guidelines in the United States are that dialysis patients should only receive gadolinium agents where essential, and that dialysis should be performed as soon as possible after the scan to remove the agent from the body promptly.[44] In Europe, where more gadolinium-containing agents are available, a classification of agents according to potential risks has been released.[45][46] Recently a new contrast agent named gadoxetate, brand name Eovist (US) or Primovist (EU), was approved for diagnostic use: this has the theoretical benefit of a dual excretion path.[47]
Pregnancy
No effects of MRI on the fetus have been demonstrated.[48] In particular, MRI avoids the use of ionizing radiation, to which the fetus is particularly sensitive. However, as a precaution, current guidelines recommend that pregnant women undergo MRI only when essential. This is particularly the case during the first trimester of pregnancy, as organogenesis takes place during this period. The concerns in pregnancy are the same as for MRI in general, but the fetus may be more sensitive to the effects—particularly to heating and to noise. However, one additional concern is the use of contrast agents; gadolinium compounds are known to cross the placenta and enter the fetal bloodstream, and it is recommended that their use be avoided.
Despite these concerns, MRI is rapidly growing in importance as a way of diagnosing and monitoring congenital defects of the fetus because it can provide more diagnostic information than ultrasound and it lacks the ionizing radiation of CT. MRI without contrast agents is the imaging mode of choice for pre-surgical, in-utero diagnosis and evaluation of fetal tumors, primarily teratomas, facilitating open fetal surgery, other fetal interventions, and planning for procedures (such as the EXIT procedure) to safely deliver and treat babies whose defects would otherwise be fatal.
Claustrophobia and discomfort
Due to the construction of some MRI scanners, they can be potentially unpleasant to lie in. Older models of closed bore MRI systems feature a fairly long tube or tunnel. The part of the body being imaged must lie at the center of the magnet, which is at the absolute center of the tunnel. Because scan times on these older scanners may be long (occasionally up to 40 minutes for the entire procedure), people with even mild claustrophobia are sometimes unable to tolerate an MRI scan without management. Modern scanners may have larger bores (up to 70 cm) and scan times are shorter. This means that claustrophobia is less of an issue, and many patients now find MRI an innocuous and easily tolerated procedure.
Nervous patients may still find the following strategies helpful:
- Advance preparation
- visiting the scanner to see the room and practice lying on the table
- visualization techniques
- chemical sedation
- general anesthesia
- Coping while inside the scanner
- holding a "panic button"
- closing eyes as well as covering them (e.g. washcloth, eye mask)
- listening to music on headphones or watching a movie with a Head-mounted display while in the machine
Alternative scanner designs, such as open or upright systems, can also be helpful where these are available. Though open scanners have increased in popularity, they produce inferior scan quality because they operate at lower magnetic fields than closed scanners. However, commercial 1.5 tesla open systems have recently become available, providing much better image quality than previous lower field strength open models.[49]
For babies and young children chemical sedation or general anesthesia are the norm, as these subjects cannot be instructed to hold still during the scanning session. Obese patients and pregnant women may find the MRI machine to be a tight fit. Pregnant women may also have difficulty lying on their backs for an hour or more without moving.
Guidance
Safety issues, including the potential for biostimulation device interference, movement of ferromagnetic bodies, and incidental localized heating, have been addressed in the American College of Radiology's White Paper on MR Safety, which was originally published in 2002 and expanded in 2004. The ACR White Paper on MR Safety has been rewritten and was released early in 2007 under the new title ACR Guidance Document for Safe MR Practices.
In December 2007, the Medicines in Healthcare product Regulation Agency (MHRA), a UK healthcare regulatory body, issued their Safety Guidelines for Magnetic Resonance Imaging Equipment in Clinical Use.
In February 2008, the Joint Commission, a US healthcare accrediting organization, issued a Sentinel Event Alert #38, their highest patient safety advisory, on MRI safety issues.
In July 2008, the United States Veterans Administration, a federal governmental agency serving the healthcare needs of former military personnel, issued a substantial revision to their MRI Design Guide, which includes physical or facility safety considerations.
The European Physical Agents Directive
The European Physical Agents (Electromagnetic Fields) Directive is legislation adopted in European legislature. Originally scheduled to be required by the end of 2008, each individual state within the European Union must include this directive in its own law by the end of 2012. Some member nations passed complying legislation and are now attempting to repeal their state laws in expectation that the final version of the EU Physical Agents Directive will be substantially revised prior to the revised adoption date.
The directive applies to occupational exposure to electromagnetic fields (not medical exposure) and was intended to limit workers’ acute exposure to strong electromagnetic fields, as may be found near electricity substations, radio or television transmitters or industrial equipment. However, the regulations impact significantly on MRI, with separate sections of the regulations limiting exposure to static magnetic fields, changing magnetic fields and radio frequency energy. Field strength limits are given, which may not be exceeded. An employer may commit a criminal offense by allowing a worker to exceed an exposure limit, if that is how the Directive is implemented in a particular member state.
The Directive is based on the international consensus of established effects of exposure to electromagnetic fields, and in particular the advice of the European Commissions's advisor, the International Commission on Non-Ionizing Radiation Protection (ICNIRP). The aims of the Directive, and the ICNIRP guidelines it is based on, are to prevent exposure to potentially harmful fields. The actual limits in the Directive are very similar to the limits advised by the Institute of Electrical and Electronics Engineers, with the exception of the frequencies produced by the gradient coils, where the IEEE limits are significantly higher.
Many Member States of the EU already have either specific EMF regulations or (as in the UK) a general requirement under workplace health and safety legislation to protect workers against electromagnetic fields. In almost all cases the existing regulations are aligned with the ICNIRP limits so that the Directive should, in theory, have little impact on any employer already meeting their legal responsibilities.
The introduction of the Directive has brought to light an existing potential issue with occupational exposures to MRI fields. There are at present very few data on the number or types of MRI practice that might lead to exposures in excess of the levels of the Directive.[50][51] There is a justifiable concern amongst MRI practitioners that if the Directive were to be enforced more vigorously than existing legislation, the use of MRI might be restricted, or working practices of MRI personnel might have to change.
In the initial draft a limit of static field strength to 2 T was given. This has since been removed from the regulations, and whilst it is unlikely to be restored as it was without a strong justification, some restriction on static fields may be reintroduced after the matter has been considered more fully by ICNIRP. The effect of such a limit might be to restrict the installation, operation and maintenance of MRI scanners with magnets of 2 T and stronger. As the increase in field strength has been instrumental in developing higher resolution and higher performance scanners, this would be a significant step back. This is why it is unlikely to happen without strong justification.
Individual government agencies and the European Commission have now formed a working group to examine the implications on MRI and to try to address the issue of occupational exposures to electromagnetic fields from MRI.
Three-dimensional (3D) image reconstruction
The principle
Because contemporary MRI scanners offer isotropic, or near isotropic, resolution, display of images does not need to be restricted to the conventional axial images. Instead, it is possible for a software program to build a volume by 'stacking' the individual slices one on top of the other. The program may then display the volume in an alternative manner.
3D rendering techniques
- Surface rendering
- A threshold value of greyscale density is chosen by the operator (e.g. a level that corresponds to fat). A threshold level is set, using edge detection image processing algorithms. From this, a 3-dimensional model can be constructed and displayed on screen. Multiple models can be constructed from various different thresholds, allowing different colors to represent each anatomical component such as bone, muscle, and cartilage. However, the interior structure of each element is not visible in this mode of operation.
- Volume rendering
- Surface rendering is limited in that it only displays surfaces that meet a threshold density, and only displays the surface closest to the imaginary viewer. In volume rendering, transparency and colors are used to allow a better representation of the volume to be shown in a single image - e.g. the bones of the pelvis could be displayed as semi-transparent, so that even at an oblique angle, one part of the image does not conceal another.
Image segmentation
Where different structures have similar threshold density, it can become impossible to separate them simply by adjusting volume rendering parameters. The solution is called segmentation, a manual or automatic procedure that can remove the unwanted structures from the image.
2003 Nobel Prize
Reflecting the fundamental importance and applicability of MRI in medicine, Paul Lauterbur of the University of Illinois at Urbana-Champaign and Sir Peter Mansfield of the University of Nottingham were awarded the 2003 Nobel Prize in Physiology or Medicine for their "discoveries concerning magnetic resonance imaging". The Nobel citation acknowledged Lauterbur's insight of using magnetic field gradients to determine spatial localization, a discovery that allowed rapid acquisition of 2D images. Mansfield was credited with introducing the mathematical formalism and developing techniques for efficient gradient utilization and fast imaging. The actual research that won the prize was done almost 30 years before, while Paul Lauterbur was at Stony Brook University in New York.
The award was vigorously protested by Raymond Vahan Damadian, founder of FONAR Corporation, who claimed that he invented the MRI,[3] and that Lauterbur and Mansfield had merely refined the technology.[52] An ad hoc group, called "The Friends of Raymond Damadian", took out full-page advertisements in the New York Times and The Washington Post entitled "The Shameful Wrong That Must Be Righted", demanding that he be awarded at least a share of the Nobel Prize.[53] Also, even earlier, in the Soviet Union, Vladislav Ivanov filed (in 1960) a document with the USSR State Committee for Inventions and Discovery at Leningrad for a Magnetic Resonance Imaging device,[54] although this was not approved until the 1970s.[55] In a letter to Physics Today, Herman Carr pointed out his own even earlier use of field gradients for one-dimensional MR imaging.[56]
See also
|
|
|
References
- ↑ Squire LF, Novelline RA (1997). Squire's fundamentals of radiology (5th ed.). Harvard University Press. ISBN 0-674-83339-2.
- ↑ Lauterbur PC (1973). "Image Formation by Induced Local Interactions: Examples of Employing Nuclear Magnetic Resonance". Nature 242: 190–191. doi:10.1038/242190a0.
- ↑ 3.0 3.1 3.2 Filler AG (2010). "The history, development, and impact of computed imaging in neurological diagnosis and neurosurgery: CT, MRI, DTI". Internet Journal of Neurosurgery 7 (1).
- ↑ Lauterbur PC (1974). "Magnetic resonance zeugmatography". Pure and Applied Chemistry 40: 149–157. doi:10.1351/pac197440010149.
- ↑ Damadian R, Goldsmith M, Minkoff L (1977). "NMR in cancer: XVI. Fonar image of the live human body". Physiological Chemistry and Physics 9: 97–100.
- ↑ Hinshaw DS, Bottomley PA, Holland GN (1977). "Radiographic thin-section image of the human wrist by nuclear magnetic resonance". Nature 270: 722–723. doi:10.1038/270722a0.
- ↑ doi:10.1080/000164800750000270
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ doi:10.1007/s00399-004-0401-5
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. (November 1986). "MR imaging of intravoxel incoherent motions: Application to diffusion and perfusion in neurologic disorders.". Radiology. 161 (2): 401–7. PMID 3763909.
- ↑ Moseley ME, Cohen Y, Mintorovitch J, Chileuitt L, Shimizu H, Kucharczyk J, Wendland MF, Weinstein PR. (1990). "Early detection of regional cerebral ischemia in cats: Comparison of diffusion- and T2-weighted MRI and spectroscopy". Magn Reson Med 14 (2): 330–46. doi:10.1002/mrm.1910140218. PMID 2345513.
- ↑ De Coene B, Hajnal JV, Gatehouse P, Longmore DB, White SJ, Oatridge A, Pennock JM, Young IR, Bydder GM. (November 1992). "MR of the brain using fluid-attenuated inversion recovery (FLAIR) pulse sequences". Am J Neuroradiol 13 (6): 1555–64. PMID 1332459.
- ↑ Haacke, E Mark; Brown, Robert F; Thompson, Michael; Venkatesan, Ramesh (1999). Magnetic resonance imaging: Physical principles and sequence design. New York: J. Wiley & Sons. ISBN 0-471-35128-8.
- ↑ Ridgway JP, Smith MA (June 1986). "A technique for velocity imaging using magnetic resonance imaging". Br J Radiol 59 (702): 603–7. doi:10.1259/0007-1285-59-702-603. PMID 3708269.
- ↑ Njemanze PC, Beck OJ (1989). "MR-gated intracranial CSF dynamics: Evaluation of CSF pulsatile flow". AJNR Am J Neuroradiol 10 (1): 77–80. PMID 2492733.
- ↑ Rosen Y, Lenkinski RE (2007). "The Recent advances in magnetic resonance neurospectroscopy.". Neurotherapeutics 27 (3): 330–45. doi:10.1016/j.nurt.2007.04.009. PMID 17599700.
- ↑ Golder W (2007). "Magnetic resonance spectroscopy in clinical oncology.". Onkologie 27 (3): 304–9. doi:10.1159/000077983. PMID 15249722.
- ↑ Thulborn KR, Waterton JC, Matthews PM, Radda GK (February 1982). "Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field". Biochim. Biophys. Acta 714 (2): 265–70. PMID 6275909. http://linkinghub.elsevier.com/retrieve/pii/0304-4165(82)90333-6.
- ↑ Wattjes MP, Lutterbey GG, Gieseke J, et al. (1 January 2007). "Double inversion recovery brain imaging at 3T: Diagnostic value in the detection of multiple sclerosis lesions". AJNR Am J Neuroradiol 28 (1): 54–9. PMID 17213424. http://www.ajnr.org/cgi/pmidlookup?view=long&pmid=17213424.
- ↑ Nelson F, Poonawalla AH, Hou P, Huang F, Wolinsky JS, Narayana PA (October 2007). "Improved identification of intracortical lesions in multiple sclerosis with phase-sensitive inversion recovery in combination with fast double inversion recovery MR imaging". AJNR Am J Neuroradiol 28 (9): 1645–9. doi:10.3174/ajnr.A0645. PMID 17885241.
- ↑ Nelson F, Poonawalla A, Hou P, Wolinsky JS, Narayana PA (November 2008). "3D MPRAGE improves classification of cortical lesions in multiple sclerosis". Mult Scler. 14 (9): 1214–9. doi:10.1177/1352458508094644. PMID 18952832.
- ↑ Brant-Zawadzki M, Gillan GD, Nitz WR (March 1992). "MP RAGE: A three-dimensional, T1-weighted, gradient-echo sequence—initial experience in the brain". Radiology 182 (3): 769–75. PMID 1535892. http://radiology.rsnajnls.org/cgi/pmidlookup?view=long&pmid=1535892.
- ↑ "Terranova-MRI Earth's Field MRI teaching system". Magritek.com. http://www.magritek.com/terranova.html. Retrieved 2010-08-02.
- ↑ I. M. Savukov and M. V. Romalis (2005). "MNR Detection with an Atomic Magnetometer" (PDF). Physical Review Letters 94. http://www.atomic.princeton.edu/romalis/magnetometer/papers/Savukov%20and%20Romalis%20-%20NMR%20Detection%20with%20an%20Atomic%20Magnetometer.pdf. Blog comment:
- "Hi-res, cheap & portable MRI". Neurophilosophy (blog). http://neurophilosophy.wordpress.com/2006/09/06/hi-res-cheap-portable-mri/.
- ↑ Raftery D (August 2006). "MRI without the magnet". Proc Natl Acad Sci USA. 103 (34): 12657–8. doi:10.1073/pnas.0605625103. PMID 16912110.
- ↑ Wu Y, Chesler DA, Glimcher MJ, et al. (February 1999). "Multinuclear solid-state three-dimensional MRI of bone and synthetic calcium phosphates". Proc. Natl. Acad. Sci. U.S.A. 96 (4): 1574–8. doi:10.1073/pnas.96.4.1574. PMID 9990066. PMC 15521. http://www.pnas.org/cgi/pmidlookup?view=long&pmid=9990066.
- ↑ Mietchen, D.; Aberhan, M.; Manz, B.; Hampe, O.; Mohr, B.; Neumann, C.; Volke, F. (2008). "Three-dimensional Magnetic Resonance Imaging of fossils across taxa". Biogeosciences 5 (1): 25–41. doi:10.5194/bg-5-25-2008. http://direct.sref.org/1726-4189/bg/2008-5-25. Retrieved 2008-04-08.
- ↑ Colosimo C, Celi G, Settecasi C, Tartaglione T, Di Rocco C, Marano P. (1995 October). "Magnetic resonance and computerized tomography of posterior cranial fossa tumors in childhood. Differential diagnosis and assessment of lesion extent" (in Italian). Radiol Med (Torino) 90 (4): 386–95. PMID 8552814.
- ↑ Allen ED, Byrd SE, Darling CF, Tomita T, Wilczynski MA. (1993). "The clinical and radiological evaluation of primary brain neoplasms in children, Part II: Radiological evaluation.". J Natl Med Assoc. 85 (7): 546–53. PMID 8350377.
- ↑ Deck MD, Henschke C, Lee BC, Zimmerman RD, Hyman RA, Edwards J, Saint Louis LA, Cahill PT, Stein H, Whalen JP. (March 1989). "Computed tomography versus magnetic resonance imaging of the brain. A collaborative interinstitutional study". Clin Imaging 13 (1): 2–15. doi:10.1016/0899-7071(89)90120-4. PMID 2743188.
- ↑ Stamford Hospital price quotation October 2008, Stamford CT US
- ↑ Gordon AC, et al "Over-utilization of MRI in the osteoarthritis patient" AAOS meeting 2008; P145.
- ↑ "LFMI - Home Page". Lfmi.ninds.nih.gov. http://www.lfmi.ninds.nih.gov/img117arrive.php. Retrieved 2010-08-02.
- ↑ 33.0 33.1 Randal C. Archibold, "Hospital Details Failures Leading to M.R.I. Fatality", The New York Times, August 22, 2001
- ↑ 34.0 34.1 Donald G. McNeil Jr, "M.R.I.'s Strong Magnets Cited in Accidents ", The New York Times, August 19, 2005.
- ↑ "ACR Guidance Document for Safe MR Practices: 2007". http://www.acr.org/SecondaryMainMenuCategories/quality_safety/MRSafety/safe_mr07.aspx. Retrieved 2 August 2010.
- ↑ "MRI Design Guide". http://www.Mednovus.com/downloads/VA_MRI_Design_Guide-08.pdf. Retrieved 2 August 2010.
- ↑ Price DL, de Wilde JP, Papadaki AM, Curran JS, Kitney RI (January 2001). "Investigation of acoustic noise on 15 MRI scanners from 0.2 T to 3 T.". Journal of Magnetic Resonance Imaging 13 (2): 288–293. doi:10.1002/1522-2586(200102)13:2<288::AID-JMRI1041>3.0.CO;2-P. PMID 11169836.
- ↑ The Open University 2007: Understanding Cardiovascular Diseases, course book for the lesson SK121 Understanding cardiovascular diseases, printed by University Press, Cambridge, ISBN 9780749226770 (can be found at OUW), pages 220 and 224.
- ↑ Kanal E, Barkovich AJ, Bell C, et al. (2007). "ACR Guidance Document for Safe MR Practices: 2007". AJR Am J Roentgenol. 188 (6): 1–27. doi:10.2214/AJR.06.1616. PMID 17515363. http://www.acr.org/SecondaryMainMenuCategories/quality_safety/MRSafety/safe_mr07.aspx. page 22.
- ↑ International Electrotechnical Commission 2008: Medical Electrical Equipment - Part 2-33: Particular requirements for basic safety and essential performance of magnetic resonance equipment for medical diagnosis, manufacturers' trade standards [1], published by International Electrotechnical Commission, ISBN 2-8318-9626-6 (can be found for purchase at [2]).
- ↑ Murphy KJ, Brunberg JA, Cohan RH (1 October 1996). "Adverse reactions to gadolinium contrast media: A review of 36 cases". AJR Am J Roentgenol 167 (4): 847–9. PMID 8819369. http://www.ajronline.org/cgi/pmidlookup?view=long&pmid=8819369.
- ↑ "ACR guideline, 2005"
- ↑ H.S. Thomsen, S.K. Morcos and P. Dawson (November 2006). "Is there a causal relation between the administration of gadolinium-based contrast media and the development of nephrogenic systemic fibrosis (NSF)?". Clinical Radiology 61 (11): 905–6. doi:10.1016/j.crad.2006.09.003. PMID 17018301.
- ↑ "FDA Public Health Advisory: Gadolinium-containing Contrast Agents for Magnetic Resonance Imaging"
- ↑ [3]
- ↑ "ismrm.org MRI Questions and Answers" (PDF). http://www.ismrm.org/special/EMEA2.pdf. Retrieved 2010-08-02.
- ↑ "Response to the FDA's May 23, 2007, Nephrogenic Systemic Fibrosis Update1 — Radiology". Radiology.rsna.org. 2007-09-12. http://radiology.rsna.org/content/246/1/11.full?searchid=1&HITS=10&hits=10&sortspec=relevance&resourcetype=HWCIT&maxtoshow=&RESULTFORMAT=&author1=kanal&FIRSTINDEX=0. Retrieved 2010-08-02.
- ↑ Ibrahim A. Alorainy, Fahad B. Albadr, Abdullah H. Abujamea (2006). "Attitude towards MRI safety during pregnancy". Ann Saudi Med 26 (4): 306–9. PMID 16885635. http://www.saudiannals.net/pdfs/06-201.pdf.
- ↑ "Siemens Introduces First 1.5 Tesla Open Bore MRI". Medical.siemens.com. 2004-07-29. http://www.medical.siemens.com/webapp/wcs/stores/servlet/PressReleaseView~q_catalogId~e_-1~a_catTree~e_100005,13839,17712~a_langId~e_-1~a_pageId~e_50677~a_storeId~e_10001.htm. Retrieved 2010-08-02.
- ↑ Bassen, H; Schaefer, D J. ; Zaremba, L; Bushberg, J; Ziskin, M [S]; Foster, K R. (2005). "IEEE Committee on Man and Radiation (COMAR) technical information statement "Exposure of medical personnel to electromagnetic fields from open magnetic resonance imaging systems"". Health Physics 89 (6): 684–9. doi:10.1097/01.HP.0000172545.71238.15. PMID 16282801.
- ↑ HSE 2007,RR570:Assessment of electromagnetic fields around magnetic resonance (MRI) equipment. MCL-T Ltd, London
- ↑ Filler, AG (2009). "The history, development, and impact of computed imaging in neurological diagnosis and neurosurgery: CT, MRI, DTI". Nature Precedings. doi:10.1038/npre.2009.3267.2.
- ↑ H.F. Judson (20 October 2003). "No Nobel Prize for whining". New York Times.
- ↑ MacWilliams, Bryon (2003). "News & Views: Russian claims first in magnetic imaging". Nature 426 (6965): 375. doi:10.1038/426375a. PMID 14647349.
- ↑ "Best Regards to Alfred Nobel". http://www.inauka.ru/english/article36919.html. Retrieved 2009-10-16.
- ↑ Carr, Herman (2004). "Letter: Field Gradients in Early MRI". Physics Today 57 (7): 83. doi:10.1063/1.1784322.}
Further reading
- Simon, Merrill; Mattson, James S (1996). The pioneers of NMR and magnetic resonance in medicine: The story of MRI. Ramat Gan, Israel: Bar-Ilan University Press. ISBN 0-9619243-1-4.
- Haacke, E Mark; Brown, Robert F; Thompson, Michael; Venkatesan, Ramesh (1999). Magnetic resonance imaging: Physical principles and sequence design. New York: J. Wiley & Sons. ISBN 0-471-35128-8.
- Lee, S. C. et al., (2001). One Micrometer Resolution NMR Microscopy. J. Magn. Res., 150: 207-213.
External links
- A Guided Tour of MRI: An introduction for laypeople National High Magnetic Field Laboratory
- The Basics of MRI. Underlying physics and technical aspects.
- Video: What to Expect During Your MRI Exam from the Institute for Magnetic Resonance Safety, Education, and Research (IMRSER)
- International Society for Magnetic Resonance in Medicine
- Trends in Biotechnology Volume 28, Issue 7, July 2010, Pages 363-370
|
||||||||||||||||||||||